To Dioselina Salto, the first two weeks of motherhood were amazing, even though she spent much of it in the hospital after her daughter was born prematurely.
She visited her daughter Janelle twice a day, held her close to her own skin and pumped breast milk for her, which doctors supplemented with specialized formula.
But a midnight call from the hospital changed everything. Doctors told Salto that Janelle had developed a life-threatening intestinal disease called necrotizing enterocolitis, or NEC. Janelle underwent more than half a dozen surgeries over the course of two weeks.
“She wasn’t getting any better,” said Salto, of Yorkville. “She just got to the point where the doctors told us that there wasn’t anything else they could do for her, that that was the end for her.”
Janelle died on her one month birthday, with bottles of unused breast milk — meant to help Janelle grow — still sitting in her mother’s refrigerator
Now, years later, Salto and hundreds of other parents whose babies developed NEC are looking for change and accountability. They’re at the center of a legal battle with the makers of specialized formulas for preterm infants — north suburban-based Abbott Laboratories and Indiana-based Mead Johnson Nutrition — that could have implications for parents and babies born prematurely for years to come.
Parents have filed more than 670 lawsuits in federal court in Chicago, over 60 in the Circuit Court of Cook County and others in courts across the country. Parents allege that the specialized, cow’s-milk based products for preterm infants caused their babies to develop NEC and that Abbott and Mead Johnson failed to warn them about the risks of giving their formulas to very small, premature infants. The companies, meanwhile, say their formulas do not cause NEC, and the American Academy of Pediatrics says they provide an essential source of nutrition.
Three of the cases have gone to trial. Two resulted in verdicts against the formula companies, totaling $495 million against Abbott Laboratories and $60 million against Mead Johnson. The third case was heard in St. Louis last month, with jurors declining to find either formula company liable for damages.
The legal battles have raised questions about the science of feeding preterm infants and the responsibilities of U.S. regulators when it comes to the safety of formula products. The lawsuits have also ignited fears that the companies could stop manufacturing the specialized formulas altogether, leaving some preterm babies without access to the nutrition they may need to survive.
Mead Johnson said in a statement it has “no plans to stop providing these products.”
“Doing so would leave neonatologists without an important option for caring for the most vulnerable infants,” Mead Johnson said in the statement.
But during Abbott’s most recent earnings call, Chairman and CEO Robert Ford warned, “If the regulatory process is disregarded, if the science is disregarded, it’s going to be very difficult for any company to remain on the market with these products, taking on that indefinite liability here, at least in the United States.”
Abbott sells about $9 million in nutritional products for preterm infants each year — a tiny slice of the company’s overall $40 billion in annual sales.
If both companies pulled their preterm infant formulas off the market, “that would leave us in a very, very difficult position in taking care of (premature) babies in the U.S.,” said Dr. Eric Eichenwald, a neonatologist at Children’s Hospital of Philadelphia who is chair of the American Academy of Pediatrics Committee on Fetus and Newborn.
More than 10% of babies in the U.S. and Illinois were born premature – defined as before 37 weeks of pregnancy – in 2023, according to the Centers for Disease Control and Prevention’s natality database. About 56,000 babies were born before 32 weeks’ gestation last year, an age at which research shows they can be at higher risk of developing NEC.
Dr. Richard Carmona, who served as the 17th U.S. surgeon general under President George W. Bush, said the loss of these products could affect premature babies in many places in the country and the world.
“More research needs to be done,” Carmona said. “In the meantime we can’t abandon these babies.”
‘The science isn’t there’
It took two weeks before Elizabeth Whitfield’s son, born 13 weeks early and weighing a little more than a quart of milk, started showing signs of NEC. His tiny belly and veins ballooned and his skin became discolored.
He underwent surgery and three quarters of his small intestine had to be removed.
After multiple surgeries that removed Whitfield’s son’s right colon, ileum and more of his small intestine, he survived and is now a 7-year-old with severe, lifelong medical needs. He can’t ride a bike, get dressed or brush his teeth without assistance. He can’t eat many foods typical children consume, and picks up on things more slowly than his 3-year-old sister, his grandmother told jurors in October during testimony streamed on Courtroom View Network.
Attorneys for the family told the boy’s story in court in recent weeks as his case — the third involving formula for premature infants to go to trial — was heard. Whitfield’s son was fed Abbott and Mead Johnson’s products for premature infants more than two dozen times over the course of six days, to supplement Whitfield’s milk, before the NEC took hold.
Jurors in the case were asked to decide whether ingesting the formula caused or contributed to the boy’s NEC diagnosis and if the formula companies and the hospital should be held financially responsible for his plight. The jury decided not to hold the companies liable.
In these cases, juries are being asked to weigh in on questions about NEC that scientists say they are still trying to answer.
NEC is a terrifying disease in which tissue lining the intestine becomes inflamed and dies. Preterm and low-birth weight babies are at higher risk than full-term babies of developing NEC, potentially because of their immature digestive systems, according to the National Institutes of Health. Research indicates 15% to 40% of infants with the disease die.
The earlier a premature baby is born, the higher their risk of NEC, said Dr. Tarah Colaizy, a professor of pediatrics at the University of Iowa. The risk starts to drop significantly once a baby reaches about 32 to 34 weeks of gestational age.
Colaizy recently published a study that found the risk of NEC in preterm babies, born before 29 weeks of pregnancy, who were fed formula was twice as high as in those fed donated breast milk — a finding similar to those from numerous other past studies. The U.S. surgeon general in 2011 also acknowledged that formula feeding is associated with higher rates of NEC for premature infants.
But that’s not to say that the specialized formulas cause NEC in premature babies. Some premature babies who are fed only breast milk also develop NEC.
“We don’t know what causes NEC, which is very frustrating for neonatologists and certainly families of infants affected by this condition,” Colaizy said. “It’s obviously quite a complex condition because the exact cause and reasoning has not yet been established, despite a lot of really smart people working hard on it.”
Colaizy was part of a working group recently tasked by the head of the U.S. Department of Health and Human Services with compiling a report on the state of science related to NEC and the feeding of preterm infants. That group released its report in September, and the findings prompted three major federal agencies — the U.S. Food and Drug Administration, the CDC and the National Institutes of Health — to release a joint statement on NEC and premature infants.
In that statement, they confirmed, “There is no conclusive evidence that preterm infant formula causes NEC.”
“Available evidence supports the hypothesis that it is the absence of human milk — rather than the exposure to formula — that is associated with an increase in the risk of NEC,” according to the statement.
The three federal agencies wrote that “important scientific gaps exist” when it comes to understanding NEC and how feeding practices may relate to it.
Jennifer Canvasser, of California, started the NEC Society to help advance research and raise awareness of the disease after her son Micah developed NEC nearly 13 years ago. Micah was born prematurely and diagnosed with NEC when he was about 6 weeks old. He recovered from NEC, but suffered permanent kidney damage while fighting the illness, and ultimately his kidneys failed. He was too ill from his battle with NEC to get a kidney transplant, and he died when he was about 10 months old.
Micah’s twin, also born prematurely, did not get NEC and is now a thriving 12-year-old boy. Both of Canvasser’s children received her breast milk, with a cow’s milk-based fortifier. Breast milk must be fortified for most premature infants because they need additional nutrients for adequate growth and development. Some of the lawsuits against Abbott and Mead Johnson also raise concerns about cow’s milk-based fortifiers. But more research is needed to understand whether using human milk-based fortifiers rather than cow’s milk-based fortifiers could help to reduce the risk of NEC, according to the working group’s report.
“When Micah died, I was desperate for answers,” said Canvasser, who was also part of the working group. “I wanted to know what caused the disease. Why did he die? I wanted to have someone, something to blame.
“I share that desperate call for answers and for clarity. I think it’s just really difficult when the science isn’t there.”
‘How much money is your child worth?’
Despite the many unanswered questions about NEC, many doctors, scientists and even lawyers suing Abbott and Mead Johnson say that the formulas should not be taken off the market.
The formulas in question are different from the ones parents pick up off grocery store shelves to feed their babies at home.
Doctors use preterm formulas to transition babies from donor breast milk — which is often fed to infants if their mothers can’t provide enough milk — before they’re sent home from the hospital, Eichenwald said. They’re also sometimes given to babies who are born close to full term but who are underweight, and to preterm babies whose parents don’t want them to have donor breast milk, Colaizy said.
“There are many, many populations who benefit from preterm infant formula products who are not at risk of NEC,” Colaizy said. “I would worry that infants who were never at risk of NEC anyway will suffer nutritionally in ways that could have lifelong implications for them if the products were pulled.”
Tor Hoerman, an attorney who represented mother Margo Gill in her case against Abbott over the summer, said, “(We’re) not saying (the specialized formulas) should come off the market but they should be used when only absolutely necessary or in situations where there’s no donor milk available,” or when babies are old enough to no longer be at serious risk of developing NEC.
Over the summer, the jury delivered a $495 million verdict in Gill’s case against Abbott.
Shortly after that verdict, the president of the American Academy of Pediatrics, Dr. Benjamin Hoffman, released a statement saying: “Providing special formula is a routine and necessary part of care of these preterm infants” and “we must take steps to protect the supply of infant formula for those who need it.”
Neonatologists agree that mother’s milk should always be the first choice for preterm babies, but it’s not always available. Some mothers can’t produce milk right away, others can’t produce enough milk, and some take medications that make their breast milk unsuitable for their babies. Other mothers do not wish to breastfeed for various reasons.
Many doctors’ next choice is donated human breast milk, but that, too, may not always be available to all preterm infants — despite a sufficient national and local supply.
“There is enough donor milk for everyone who needs it” in neonatal intensive care units across the country, said Lindsay Groff, executive director of the Human Milk Banking Association of North America. Women who donate the milk must answer a questionnaire about their health and undergo a blood test that screens for certain diseases; their milk is then screened for bacterial growth, pooled with milk from other mothers and pasteurized before it’s given to babies.

The Mothers’ Milk Bank of the Western Great Lakes has enough donor milk to serve all hospitals in Illinois and Wisconsin as well as babies at home with medical needs in those states, said Summer Kelly, the bank’s executive director.
Yet not all hospitals offer donor milk to premature infants. All of the highest level NICUs in the country that responded to a 2022 CDC survey said they had donor milk available, but donor milk was not available at about 8% of the next highest level NICUs.
Though moms aren’t paid for donated milk, the milk banks must pay to run the blood tests and process the milk, so they, in turn, charge hospitals for it. The price varies by milk bank, but a 4-ounce bottle of donated milk may cost a hospital about $9 to $22, Kelly said.
In Illinois, hospitals charge patients between $1.50 and nearly $30 per ounce of donor human milk for inpatient care at some of the state’s largest health systems, depending on insurance coverage.
That’s more than hospitals may pay for preterm infant formulas. Attorneys for Abbott acknowledged in court testimony that the company tends to give its formulas for premature babies to the hospitals for free in hopes of building brand loyalty with parents and securing hospital contracts.
The cost of donated milk may be why, according to research, hospitals are less likely to utilize donor human milk if they serve a high percentage of Medicaid patients or serve a large proportion of Black or Latino/Hispanic patients. Medicaid is a state and federally funded health insurance program for people with lower incomes.
Nearly half of the babies born in 2023 before 32 weeks gestational age, facing a higher risk of developing NEC, were birthed by mothers on Medicaid, according to the CDC’s birth data. Human donor milk is an optional benefit of the federal Medicaid program, like hearing aids for most adults, that states can decide whether or not to cover.
Illinois state law requires both Medicaid and private insurers to cover the cost of human donor milk for certain babies, including those with low birth weights who are at increased risk of developing NEC. State Rep. Katie Stuart, D-Edwardsville, was behind the 2019 bill that led to that law in Illinois.
“It can be extremely expensive,” Stuart said of donor milk. In Illinois, in 2020, more than 90% of hospitals with neonatal intensive care units had donor milk available for very low birth weight babies, according to a CDC survey.
At least 26 states and the District of Columbia have similar laws or administrative rules in place, requiring that public or private health plans cover human donor milk in some circumstances, according to a Tribune review of state laws nationwide.
“If it’s not covered by insurers, many hospitals will provide it without reimbursement, but some hospitals are unable to do so because of financial situations,” said Eichenwald, chair of the American Academy of Pediatrics’ Committee on Fetus and Newborn.
In Virginia, state Sen. Jennifer Carroll Foy has twice filed bills in the statehouse that would require both the state’s Medicaid program and private insurers offering plans to cover medically necessary human donor milk. Her bill failed this February, but Carroll Foy said she is not giving up on the issue.
Virginia’s Medicaid program voluntarily covers donor milk costs while infants are in the NICU but does not pay for donor milk in other circumstances.
For Carroll Foy, it’s a deeply personal matter. In 2017 she gave birth to twins at 22 weeks. She said she didn’t even know about donor milk when her neonatologist recommended she utilize it over formula for the safety of her babies. After five months in the NICU, she said, her sons were able to survive and thrive and are now 7 years old.
Knowing the importance of breast milk, and that it isn’t covered in many instances in her state, Carroll Foy said she presented the bill to help combat infant mortality.
“How much money is your child worth?” Carroll Foy said. “How much is too much to spend for your child who is fighting for his or her life. That’s what it really comes down to.”
Legislative attempts to require Medicaid coverage of donor human milk have failed in at least eight other states, including Wisconsin and Nebraska.
Sparring over safety labels
Lawsuits filed against Abbott and Mead Johnson allege the formulas are dangerous and should have had labels warning parents of the risk of NEC. But questions remain about whether such labels would be useful, and whether labeling is the responsibility of the companies or government regulators.
“Ultimately, the regulator decides if the products are safe and they’re fit for purpose, and they decide how they’ve got to be labeled,” said Ford, Abbott’s CEO, in an October earnings call. He said he’d rather see products and labels evaluated through the regulatory process than “through uncertainty and unpredictable jury trials.”
According to the FDA’s website, the agency regulates formulas by ensuring the safe production of the products, including making sure that products meet labeling requirements. But the FDA does not approve formulas like it approves medications.
An FDA spokesperson said the agency does not comment on pending litigation and did not answer a reporter’s questions about whether it would need to approve any label changes on the formula. The spokesperson did not answer questions about general product oversight and would only provide links to information available on the FDA website.
Peter Pitts, a former associate commissioner at the FDA, said the lawsuits are addressing an issue that the formula manufacturers do not have complete control over.
“To say that these products were not appropriately labeled may or may not be true, but that is not really a circumstance that is on the back of the manufacturer,” Pitts said. “If the FDA thought these products should be relabeled, they would be relabeled.”
The companies acknowledged in court that no NEC warning exists on their products but said a warning label wouldn’t make a difference because parents often do not see the product packaging and neonatologists are already well-versed on the disease.
“The story that’s being told is somehow we are robbers and barons and trying to take away this product, and that is not the case,” said Hoerman, the attorney who represented Gill in St. Louis. “This product has a purpose, but this product should be used when medically necessary with the appropriate patient and the appropriate information given to the parents who are and should be involved in the feeding decisions.”
Some doctors and public health advocates say the government might consider helping by legally protecting manufacturers of preterm infant formulas. The U.S. government gave companies engaged in manufacturing and distributing COVID-19 vaccines immunity from liability in 2020.
“The similarity is, if you don’t protect vaccine manufacturers from litigation you don’t get vaccines,” Pitts said.
Local hospital takes action
Some Illinois hospitals aren’t waiting for the government or courts to act.
Mount Sinai Hospital on the West Side of Chicago decided in 2019 to only feed human milk, not formula, to babies born at less than 32 weeks’ gestation or weighing less than 1,500 grams. That means if a mother’s milk isn’t available for a baby fitting those criteria, the infant is fed donor breast milk. The hospital made the change because of the association between formula feeding and higher rates of NEC among premature infants.
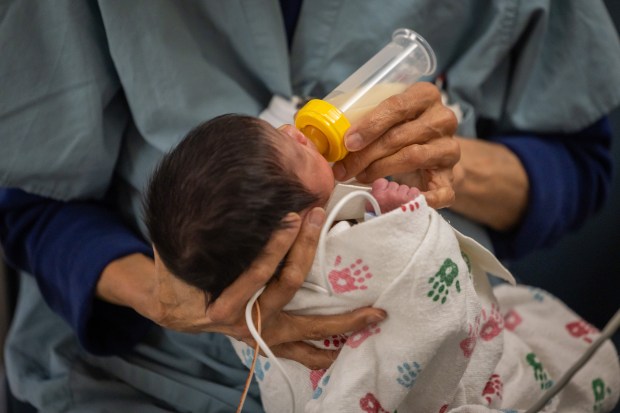
Since making the switch about five years ago, the hospital has not had a single case of severe NEC among the more than 160 very low birth weight or very premature babies it’s treated in its neonatal intensive care unit, said Dr. Hari Srinivasan, chair of the Department of Pediatrics at Mount Sinai, and an attending neonatologist at the hospital.
It’s been more expensive for the hospital to use donor milk than formula — and Mount Sinai does not serve an affluent community. About 86% of its inpatients were on Medicaid or Medicare in 2022, according to the state. But Srinivasan said the change has been worth it.
“We have to pay for the donor milk, but the benefit we’ve seen is phenomenal,” Srinivasan said. “The benefit completely outweighs the cost.”
Srinivasan said, so far, only one parent has refused donor milk for a premature baby, and, in that case, the hospital gave the baby formula. The hospital uses cow’s milk-based fortifiers, he said.
Advocate Children’s Hospital also typically gives only mother’s milk or donor’s milk to very small, premature infants, a spokesperson said.
Other Chicago-area hospitals and health care systems including Northwestern Memorial Hospital, Lurie Children’s Hospital, Endeavor Health and Rush University Medical Center either declined to answer or did not respond to the Tribune’s questions about whether they feed formula to very small, premature infants.
Jasmine Gallardo, who gave birth to her son Hugo at Mount Sinai in September, more than three months early, said she appreciates that doctors at the hospital encouraged her to pump her own milk for Hugo. While she was pregnant, she wasn’t sure if she would breastfeed Hugo. But then doctors explained the long list of health risks Hugo faced as a preterm baby.
“I was really scared,” said Gallardo, of Evergreen Park. “They tell you about everything that can happen.”

Doctors told her that her breast milk could help to protect him, so she began pumping for him, every few hours around the clock.
On a recent day, Gallardo and her mother visited Hugo in the Mount Sinai neonatal intensive care unit. A nurse gently removed Hugo from his incubator, which had been decorated with a green sign reading “NICU miracle” and a cutout of his tiny footprints at birth.
Gallardo and her mother took turns cradling Hugo, who occasionally opened his dark eyes, delighting his mother and grandmother.
Wires still snaked from the bottom of his swaddle, and a feeding tube was still running up his nostril.
But Hugo was nearly 4 weeks old, had gained nearly a pound since birth and was showing no signs of NEC.