Alzheimer’s is a progressive, fatal disease that boggled scientists for a century. In the past 30 years, $42 billion has been invested in research and development of drugs that could treat it. More than 150 trials ended in failure. Now, at last, we have two treatments with significant clinical benefit — one, lecanemab, approved by the Food and Drug Administration and another, donanemab, recently endorsed by its advisory panel — and a scientific pathway that could one day point the way toward a cure.
Unfortunately, it’s not clear how quickly patients and their families will be able to benefit. It could be months before the FDA approves donanemab. These drugs work best at early stages of this progressive disease. Patients who could see results from treatment today may not be eligible tomorrow. Our agencies must refocus their efforts on ensuring safe, appropriate access for people who may benefit from the first new treatments for Alzheimer’s in three decades.
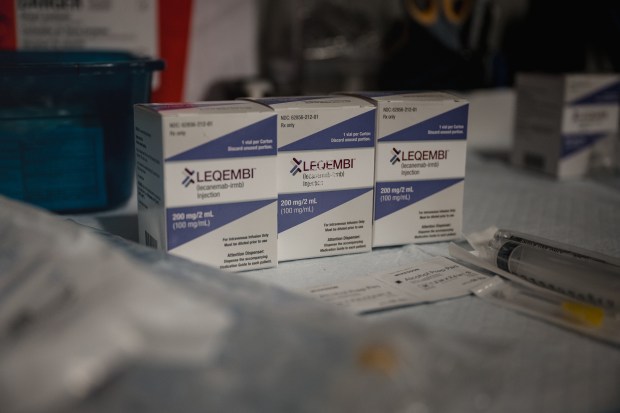
Eisai’s Leqembi (lecanemab) received FDA approval more than a year ago after demonstrating a 27% decline in early disease progression; donanemab achieved 35%. But rather than cover the drug for seniors who need it, the Centers for Medicare and Medicaid Services (CMS), which administers Medicare, restricted access, citing the very safety concerns the FDA scrutinized before granting approval.
The result? After more than a year on the market, the drug has reached only 5,000 patients.
Though the FDA reviewed the data and determined lecanemab’s benefit outweighed the risks, CMS continues to press for more data on its clinical benefit and safety. The agency isn’t supposed to consider cost in a coverage decision, but that certainly seems to be a factor. It effectuated one of the largest premium increases in the history of the Medicare program to manage the projected budget impact of a similar Alzehimer’s drug, Aduhelm — then restricted access to it and rebalanced premiums.
The tool used to nullify these drugs is “coverage with evidence development,” or CED. This program was designed to speed new technologies to market, protect patients and spur innovation. But CMS has warped its purpose, using CED to impede access not only to Alzheimer’s treatments but to diagnostic too.
PET scans are the gold standard for diagnosing Alzheimer’s by confirming the presence of amyloid plaque in the brain. A second test is used after treatment to confirm that plaques have been removed. After tying up these tests in CED for more than a decade, CMS finally concluded last October what we’ve known for years: that the preponderance of data supports using PET scans to diagnose Alzheimer’s disease. Now we must ensure equitable access to these tools in practice. The new drugs deliver the most benefit when administered early in the progression of disease, so getting a timely, accurate diagnosis is crucial.
Yet even where a patient can get access to the drug under CED, the process is burdensome for physicians, who must enter patient data in an approved registry or study to secure Medicare coverage. This adds an uncompensated layer of bureaucracy to the management of a disease that is already very complex to diagnose and treat. CMS maintains that its digital database is easy to use, but the policy is disconnected from the reality on the ground.
Neurologists’ offices are packed trying to manage the demands of an aging population. Appointments can be backed up for more than a year. Memory centers at elite medical institutions can train staff to navigate these additional tasks, but remote facilities are struggling. The result is that fewer patients have ready access to the treatment.
Donanemab faces all the same headwinds. Let’s hope a swift FDA approval is followed by a new CMS policy that helps ensures people who need these treatments can get them.
Joe Grogan is a senior fellow at the University of Southern California’s Schaeffer Center for Health Policy & Economics and former director of the Domestic Policy Council in the White House. Grogan consults for the health care industry, including those working to develop treatments for Alzheimer’s disease.
Submit a letter, of no more than 400 words, to the editor here or email letters@chicagotribune.com.